Abstract
COVID-19 is the disease caused by SARS-CoV-2. The present hospital-based study was performed to find out prevalence of Urinary Tract Infection among COVID 19 patients. The cross-sectional study was performed with seven hundred fifty-three laboratory confirmed COVID 19 cases over six months (from 1st July to 31st December, 2020). Urine samples collected from laboratory confirmed COVID-19 cases in appropriate sterile manner and were screened for pus cells and bacteria. This was followed by plating on Mac-conkey’s agar media and 5% Sheep Blood agar media. Inoculated plates were incubated overnight in aerobic condition at 37ÅãC. Discrete colonies were further studied by Gram staining, tests for motility, battery of biochemical tests. Antibiogram was performed by disk diffusion method as per CLSI guidelines. Species confirmation and MIC (Minimum Inhibitory Concentration) values of the tested antibiotics were detected by automation. Results were analyzed according to standard statistical methods. Ninety urine samples were culture positive (11.95%). Escherichia coli was found to be the commonest pathogen, isolated in forty-three cases (47.78%) followed by Enterococcus faecalis in twenty-nine (32.22%) and Klebsiella pneumoniae subspp. pneumonia in eighteen occasions (20%). Enterococcus faecalis isolates were sensitive to Vancomycin, Linezolid and Nitrofurantoin and nineteen isolates were resistant to fluroquinolones (65.51%). Majority of the Gram-Negative isolates were susceptible to nitrofurantoin (80.32%) where as fifteen carbapenemase producers, thirteen AmpC Betalactamase producers and twenty-one Extended Spectrum Beta Lactamase (ESBL) producers have been recorded. Constant awareness regarding the antibiotic guidelines for COVID-19 cases is the need of the hour.
Prevalence of Urinary Tract Infection among Hospitalized Covid 19 Patients: A Study in Eastern India
Abstract
COVID-19 is the disease caused by SARS-CoV-2. The present hospital-based study was performed to find out prevalence of Urinary Tract Infection among COVID 19 patients. The cross-sectional study was performed with seven hundred fifty-three laboratory confirmed COVID 19 cases over six months (from 1st July to 31st December, 2020). Urine samples collected from laboratory confirmed COVID-19 cases in appropriate sterile manner and were screened for pus cells and bacteria. This was followed by plating on Mac-conkey’s agar media and 5% Sheep Blood agar media. Inoculated plates were incubated overnight in aerobic condition at 37ÅãC. Discrete colonies were further studied by Gram staining, tests for motility, battery of biochemical tests. Antibiogram was performed by disk diffusion method as per CLSI guidelines. Species confirmation and MIC (Minimum Inhibitory Concentration) values of the tested antibiotics were detected by automation. Results were analyzed according to standard statistical methods. Ninety urine samples were culture positive (11.95%). Escherichia coli was found to be the commonest pathogen, isolated in forty-three cases (47.78%) followed by Enterococcus faecalis in twenty-nine (32.22%) and Klebsiella pneumoniae subspp. pneumonia in eighteen occasions (20%). Enterococcus faecalis isolates were sensitive to Vancomycin, Linezolid and Nitrofurantoin and nineteen isolates were resistant to fluroquinolones (65.51%). Majority of the Gram-Negative isolates were susceptible to nitrofurantoin (80.32%) where as fifteen carbapenemase producers, thirteen AmpC Betalactamase producers and twenty-one Extended Spectrum Beta Lactamase (ESBL) producers have been recorded. Constant awareness regarding the antibiotic guidelines for COVID-19 cases is the need of the hour.
Introduction
COVID-19 is the disease caused by a new coronavirus called SARS-CoV- 2. WHO first learned of this new virus on 31 December 2019, following a report of a cluster of cases of ‘viral pneumonia’ in Wuhan, People’s Republic of China. The most common symptoms of COVID-19 are a triad comprised of fever, dry cough and fatigue. Other less common symptoms include loss of taste or smell, nasal congestion, conjunctivitis (also known as red eyes), sore throat, chills or dizziness, headache, muscle or joint pain, different types of skin rashes, nausea or vomiting and diarrhea. Symptoms of severe COVID-19 disease include High temperature (above 38 °C) with or without shortness of breath, loss of appetite, confusion and persistent pain or pressure in the chest. Other less common symptoms of severity are irritability, confusion, reduced consciousness (sometimes associated with seizures), anxiety, depression and sleep disorders. More severe and rare neurological complications such as strokes, brain inflammation, delirium and nerve damage are also manifested in some cases. People of all ages may experience fever with or without cough associated with shortness of breath, chest pain or loss of speech or movement.1 On the other hand, Urinary tract infection (UTI) is a collective term that describes any infection involving any part of the urinary tract, namely the kidneys, ureters, bladder and urethra. The urinary tract can be divided into the upper (kidneys and ureters) and lower tract (bladder and urethra).2 Uncomplicated lower UTI remains one of the most commonly treated infections in primary care. The urinary tract is a common source of infection in children and infants and is the most common bacterial infection in children
Objective(s)
1. Estimation the prevalence of Urinary Tract Infection among COVID 19 patients. 2. Identify the pathogenic bacteria to cause Urinary Tract Infection among COVID 19 patients.
Material and Methods
The cross-sectional study was performed with seven hundred fifty-three laboratory confirmed COVID 19
cases over six months (from 1st July to 31st December, 2020) in a COVID hospital of Eastern India.

Figure 1.
Urine samples were screened from laboratory confirmed COVID 19 cases with symptoms of Urinary Tract Infection. Urine samples collected in appropriate sterile manner were screened for pus cells and bacteria. This was followed by plating on Macconkey’s agar media (differential as well as selective media for Gram Negative bacteria) and 5% Sheep Blood agar media (Enriched media, aids to isolate both Gram Negative and Gram Positive bacteria. Inoculated plates were incubated overnight at 37◦C. Discrete colonies were further studied by Gram staining, tests for motility, battery of biochemical tests and by VITEK 2 Microbial identification system (bioMerieux) with “Advanced Expert System” (AES) to confirm the speciation. Antibiogram was performed by disk diffusion method (modified Kirby- Bauer technique) on Muller-Hinton agar and blood agar media. MIC (Minimum Inhibitory Concentration) values of the tested antibiotics were detected by VITEK 2 with “Advanced Expert System” (AES) as per CLSI guidelines with its ability to provide accurate “fingerprint” recognition of bacterial resistance mechanisms and phenotypes.7 Results were analyzed according to standard statistical methods.
Results
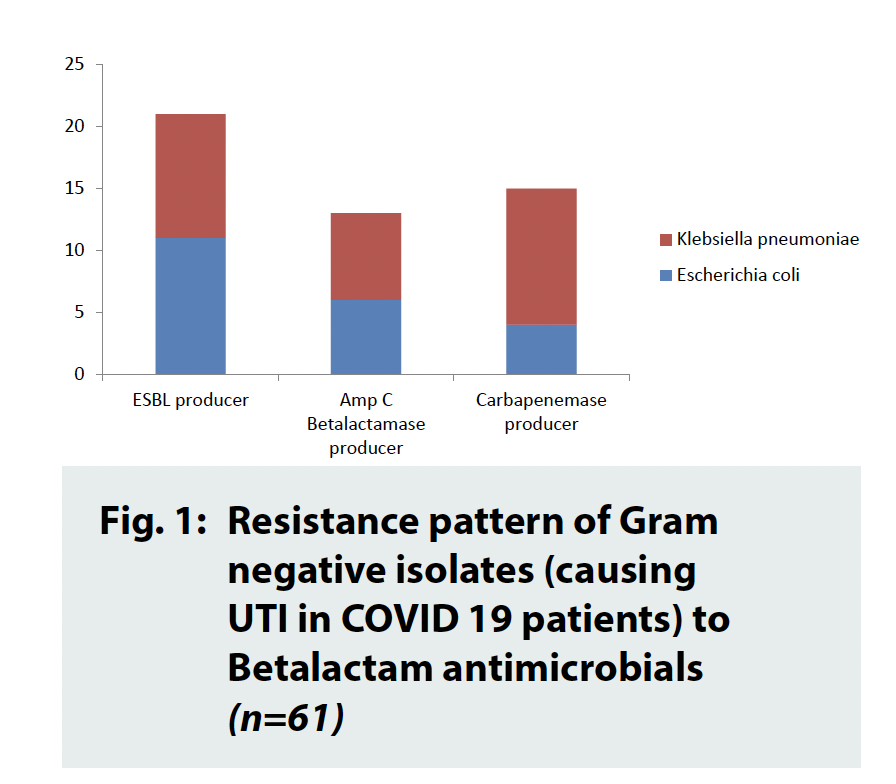
Out of seven hundred and fifty-three total samples, ninety were culture positive (11.95%). All the culture positive cases were infected by a single pathogen. Escherichia coli was found to be the commonest pathogen, isolated in forty-three cases (47.78%) followed by Enterococcus faecalis in twenty-nine times (32.22%). Klebsiella pneumoniae subspp. pneumonia was isolated in eighteen occasions (20%). No other isolate was identified. Regarding antimicrobial susceptibility pattern, all the Enterococcus faecalis isolates were sensitive to Vancomycin, Linezolid and nitrofurantoin. Out of twentynine isolates, nineteen isolates were resistant to fluroquinolones (65.51%). Majority of the Gram-Negative isolates were susceptible to nitrofurantoin (49 out of 61, i.e., 80.32%) where as fifteen carbapenemase producers, thirteen AmpC Betalactamase producers and twentyone Extended Spectrum Beta Lactamase (ESBL) producers have been recorded (Figure 1).
Discussion and Conclusion
Hospitalized COVID-19 patients have been reported to suffer from superinfection such as Ventilator Associated Pneumonia (VAP) and UTI.8,9 Similarly, in this present study, out of seven hundred and fifty-three total samples, ninety were culture positive (11.95%) UTI cases. On the other hand, few studies have documented the fact that patients admitted with COVID-19 did not have any lower urinary tract symptoms. Moreover, AJB Marand et al (2021) concluded that hematuria, pus cells and SARS- CoV-2 virus in urine, were found to be strong negative prognostic factors in admitted COVID-19 patients.6 However, several other studies such as the study performed by Sara M Karaba et al (2021)have noted that the most common urinary pathogens were Escherichia coli along with Proteus spp. and Klebsiella spp.10,11 Similarly, in the present study, Escherichia coli was found to be the commonest pathogen, isolated in forty three cases (47.78%). Higher percentage of antimicrobial resistance among the bacterial isolates causing secondary or co infection amongst the COVID 19 cases have been reported by several workers as Li J et al (2020), in accordance to the present study.12 According to the WHO proposed guidelines for the clinical management of COVID-19 cases, use of antibiotics for patients with a low suspicion of a bacterial infection is not endorsed. However, for severe suspected or confirmed COVID-19 cases, the use of empiric antimicrobials is recommended.13,14 COVID-19 viral pandemic thus has caused increased antibiotic use and also the risk of increasing antimicrobial resistance. Hence, implementation of antimicrobial stewardship is strongly recommended.
References
1. World Health Organization. Coronavirus disease (COVID- 2019) situation reports. World Health Organization Website. www.who.int/emergencies/diseases/novelcoronavirus- 2019/situation-reports.
2. Tan CW, Chlebicki MP. Urinary tract infections in adults. Singapore Med J 2016; 57:485-90. doi: 10.11622/ smedj.2016153. PMID: 27662890; PMCID: PMC5027397.
3. Hanna-Wakim RH, Ghanem ST, El Helou MW, Khafaja SA, Shaker RA, Hassan SA, et al. Epidemiology and characteristics of urinary tract infections in children and adolescents. Front Cell Infect Microbiol 2015; 5:45. doi: 10.3389/fcimb.2015.00045. PMID: 26075187; PMCID: PMC4443253.
4. Health Promotion Board Singapore Urinary tract infection. Available at: http://www.healthhub.sg/a-z/diseases-andconditions/ 210/anarytractinfection.
5. Grabe M, Bartoletti R, Bjerklund-Johansen TE, Cai T, Cek M, Koves B, et al. European Association of Urology. Guidelines on urological infections 2015.
6. Marand AJB, Bach C, Janssen D, Heesakkers J, Ghojazadeh M, Vögeli TA, et al . Lower urinary tract signs and symptoms in patients with COVID-19. BMC Infect Dis 2021; 21:706. doi: 10.1186/s12879-021-06394-z. PMID: 34311703; PMCID: PMC8312200.
7. CLSI. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline, CLSI Document No. C28-A3. 3rd ed. United States: CLSI; 2008
8. He Y, Li W, Wang Z, Chen H, Tian L, Liu D. Nosocomial infection among patients with COVID-19: a retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect Control Hosp Epidemiol 2020; 13:1–2.
9. Nag V, L, Kaur N. Superinfections in COVID-19 Patients: Role of Antimicrobials. Dubai Med J 2021; 4:117-26. 10. Vaughn VM, Gandhi TN, Petty LA, Patel PK, Prescott HC, Malani AN, et al. Empiric Antibacterial Therapy and Community-onset Bacterial Coinfection in Patients Hospitalized with Coronavirus Disease 2019 (COVID-19): A Multi-hospital Cohort Study. Clin Infect Dis 2021; 72: e533- e541. doi: 10.1093/cid/ciaa1239. PMID: 32820807; PMCID: PMC7499526.
11. Karaba SM, Jones G, Helsel T, Smith LL, Avery R, Dzintars K, et al. Prevalence of Co-infection at the Time of Hospital Admission in COVID-19 Patients, A Multicenter Study. Open Forum Infect Dis 2020; 8: ofaa578. doi: 10.1093/ofid/ofaa578. PMID: 33447639; PMCID: PMC7793465.
12. Li J, Wang J, Yang Y, Cai P, Cao J, Cai X, et al. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: a retrospective analysis. Antimicrob Resist Infect Control 2020; 9:153.
13. World Health Organization. (2020). Clinical management of COVID-19: interim guidance, 27 May 2020. World Health Organization. https://apps.who.int/iris/ handle/10665/332196.
14. Lucien MAB, Canarie MF, Kilgore PE, Jean-Denis G, Fénélon N, Pierre M, et al. Antibiotics and antimicrobial resistance in the COVID-19 era: Perspective from resource-limited settings. Int J Infect Dis 2021; 104:250-254. doi: 10.1016/j. ijid.2020.12.087. Epub 2021 Jan 9. PMID: 33434666; PMCID: PMC7796801.